Write a chemical equation for the reaction that occurs between stearic acid and triethanolamine under the conditions of the experiment. How does the product of this reaction promote the formation of the
By A Mystery Man Writer
Last updated 08 Jul 2024


Interaction of the Acid Soap of Triethanolamine Stearate and Stearic Acid with Water
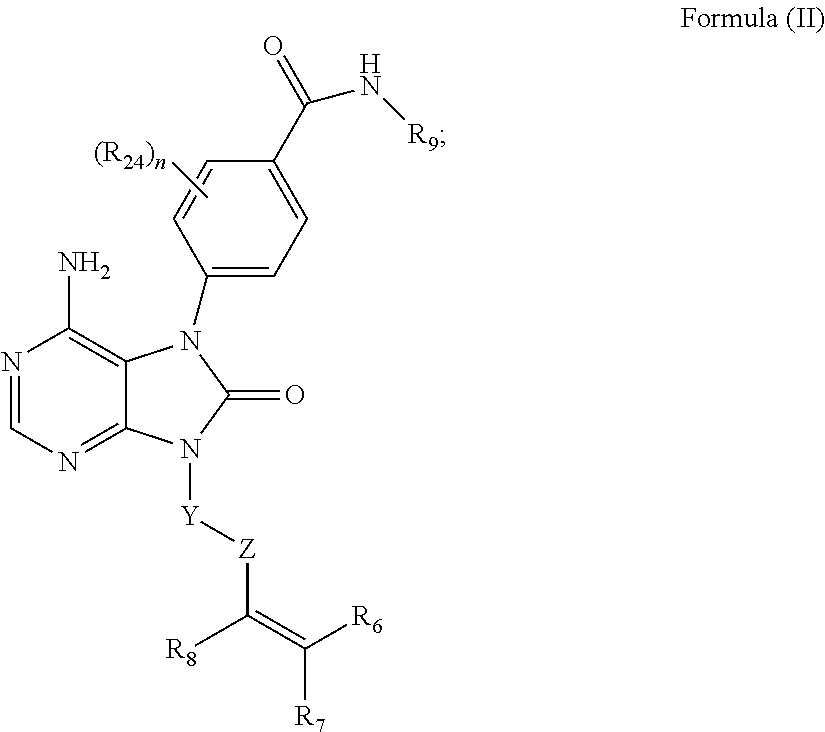
Purinone Compounds As Kinase Inhibitors Chen; Wei ; et al. [Pharmacyclics LLC]

Reactions Exam Flashcards

Solved 8. a) Is stearic acid a necessary ingredient for the

SOLVED: c) Write a chemical equation for the reaction that occurs between stearic acid and triethanolamine under the conditions of the experiment: How does the product of this reaction promote the formation

Write an equation for the full hydrogenation of glyceryl

SOLVED: c) Write a chemical equation for the reaction that occurs between stearic acid and triethanolamine under the conditions of the experiment: How does the product of this reaction promote the formation

Polymers, Free Full-Text

ES2702798T3 - Esteraminas and derivatives of the metathesis of natural oil - Google Patents
What are good 'natural' alternative ingredients to dimethicone and TEA ( triethanolamine) for cosmetic formulations of lotions and creams? - Quora

Principles of Advanced Biochemistry Complete Notes on Lecture Material and Tutorial Notes, BIOC2101 - Principles of Biochemistry (Advanced) - UNSW

Interaction of the Acid Soap of Triethanolamine Stearate and Stearic Acid with Water

CHEMISTRY
Recommended for you
You may also like
















![Recycled] TREE FREE (92) 8.5 X 11 White Copy Paper (10 Reams/Case)](https://www.bazicproducts.com/wp-content/uploads/2022/07/41166.jpg)

