Pharmaceutics, Free Full-Text
By A Mystery Man Writer
Last updated 17 Jul 2024
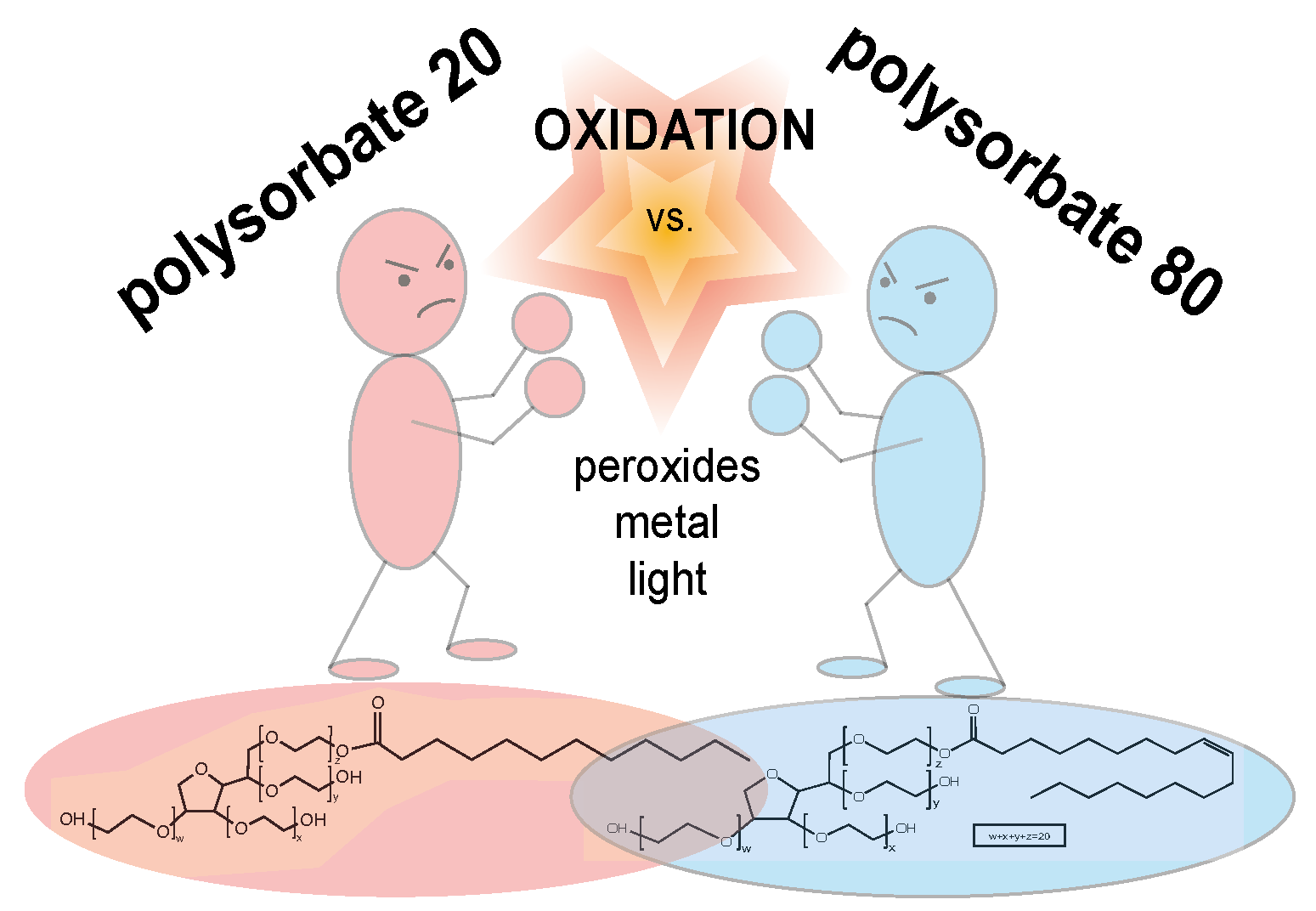
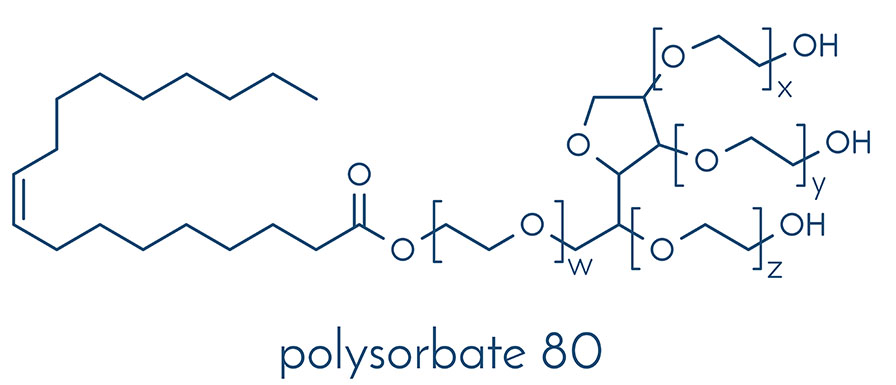
The surfactants polysorbate 20 (PS20) and polysorbate 80 (PS80) are utilized to stabilize protein drugs. However, concerns have been raised regarding the degradation of PSs in biologics and the potential impact on product quality. Oxidation has been identified as a prevalent degradation mechanism under pharmaceutically relevant conditions. So far, a systematic stability comparison of both PSs under pharmaceutically relevant conditions has not been conducted and little is known about the dependence of oxidation on PS concentration. Here, we conducted a comparative stability study to investigate (i) the different oxidative degradation propensities between PS20 and PS80 and (ii) the impact of PS concentration on oxidative degradation. PS20 and PS80 in concentrations ranging from 0.1 mg⋅mL−1 to raw material were stored at 5, 25, and 40 °C for 48 weeks in acetate buffer pH 5.5 and water, respectively. We observed a temperature-dependent oxidative degradation of the PSs with strong (40 °C), moderate (25 °C), and weak/no degradation (5 °C). Especially at elevated temperatures such as 40 °C, fast oxidative PS degradation processes were detected. In this case study, a stronger degradation and earlier onset of oxidation was observed for PS80 in comparison to PS20, detected via the fluorescence micelle assay. Additionally, degradation was found to be strongly dependent on PS concentration, with significantly less oxidative processes at higher PS concentrations. Iron impurities, oxygen in the vial headspaces, and the pH values of the formulations were identified as the main contributing factors to accelerate PS oxidation.

Hawaii Pharm Ginger (Zingiber officinale) Liquid Extract 32 oz Unfiltered : Health & Household
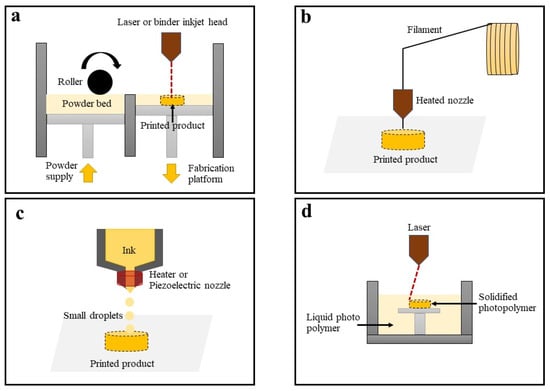
Ozeki Ng Sms Gateway Trial To Full Patched Cracked - Colaboratory
The Compendium of Pharmaceuticals and Specialties as a Drug Information Resource for Treatment of Acute Drug Overdose - Abstract - Europe PMC

Pharmaceutics, Free Full-Text, llll k

Pharmaceutics, Free Full-Text
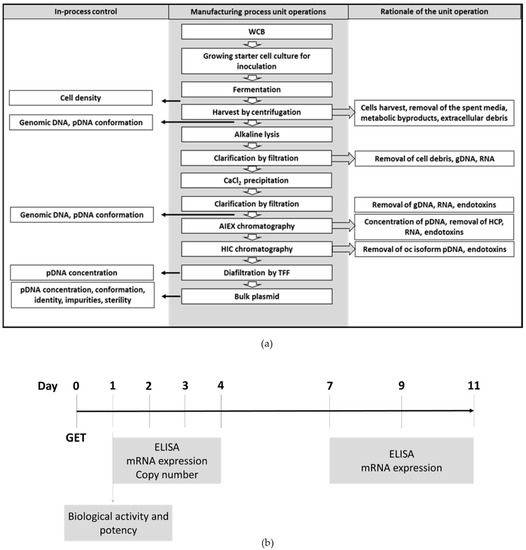
Pharmaceutics, Free Full-Text, labetalol dose

SOLUTION: Unit 1 pharmaceutics b pharmacy 1st sem - Studypool
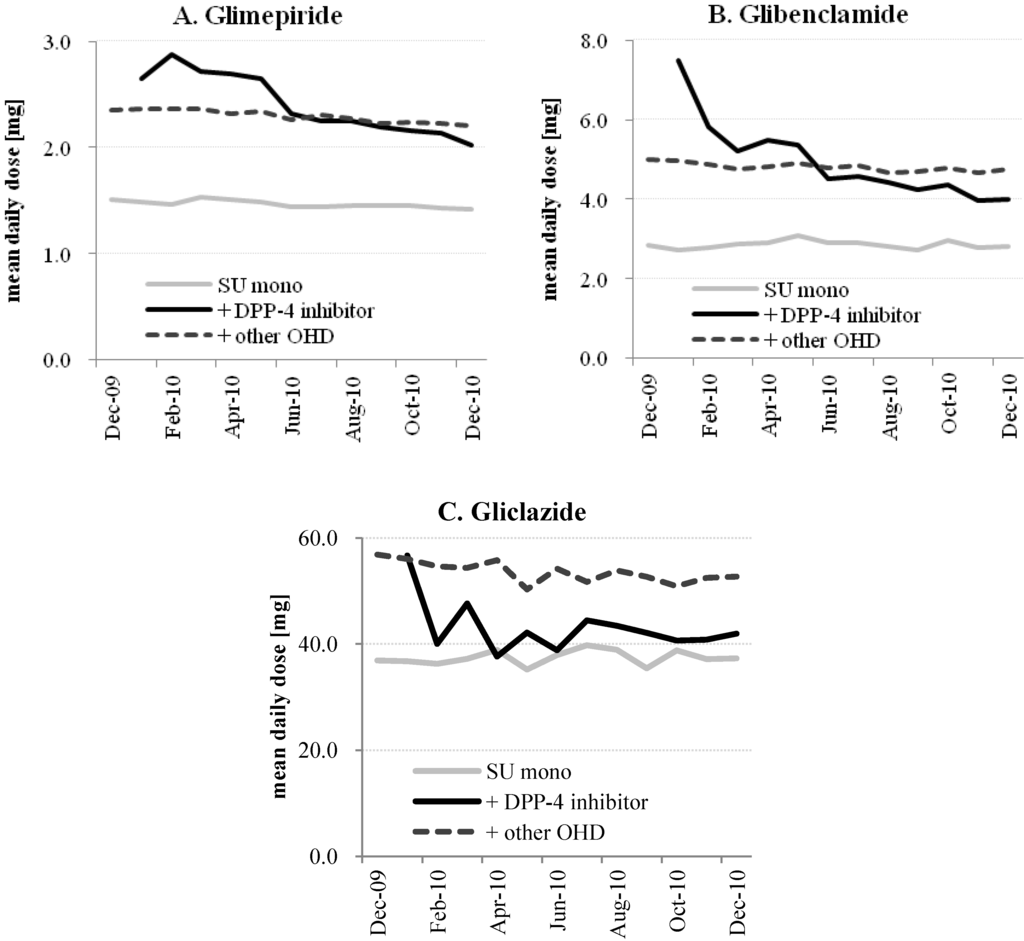
Pharmaceutics, Free Full-Text

International Journal of Pharmaceutics

Pharmaceutics, Free Full-Text, renato ruiz pacheco

Pharmaceutics Free Full-Text Drug Delivery Systems For, 56% OFF

Pharmaceutics, Free Full-Text
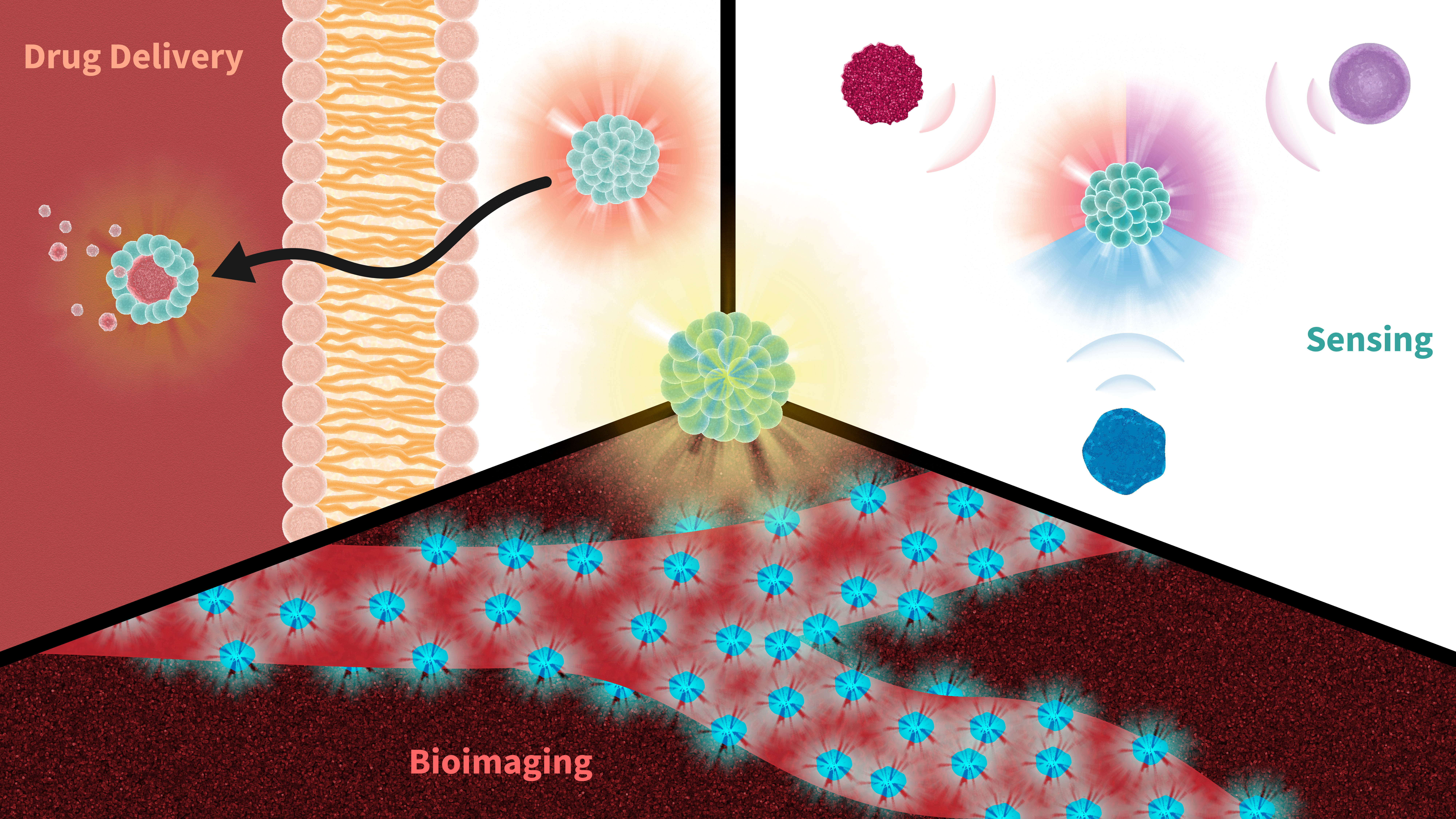
Pharmaceutics, Free Full-Text
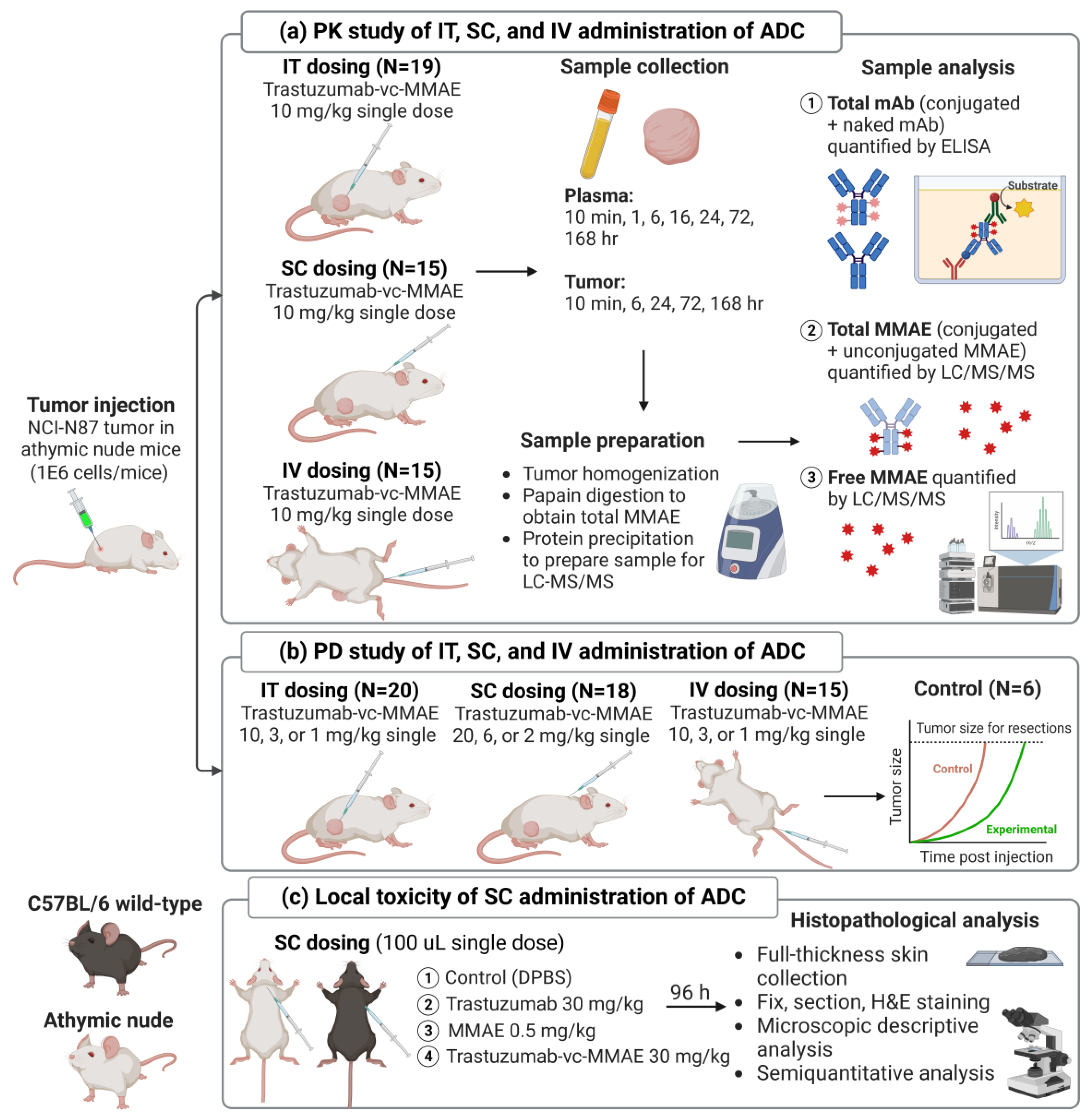
Engineering Intravenously Administered Nanoparticles to Reduce Infusion Reaction and Stop Bleeding in a Large Animal Model of Trauma
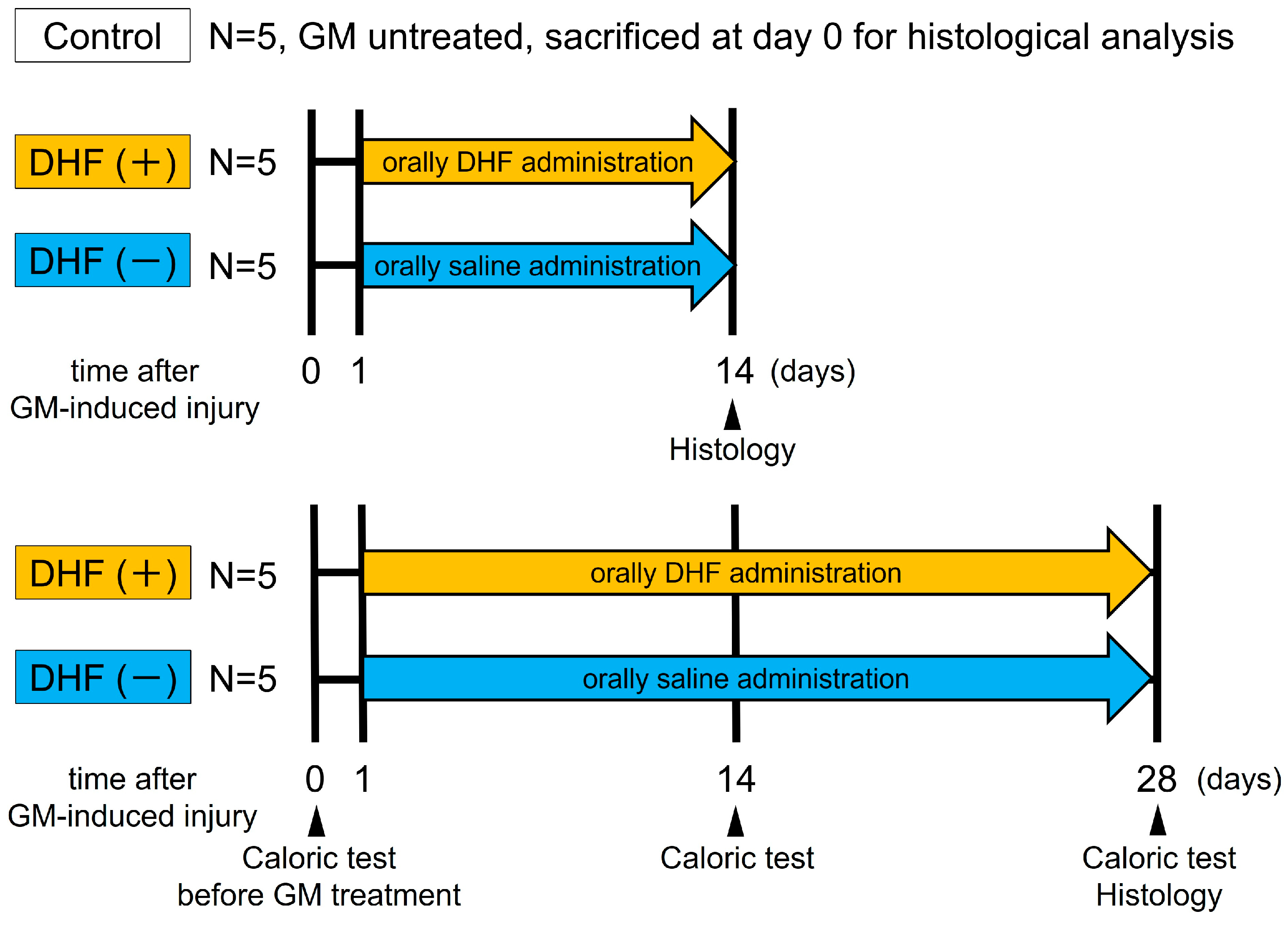
Pharmaceutics, Free Full-Text
Recommended for you
You may also like
















/images/APRIL/optical_illusions.jpg)

