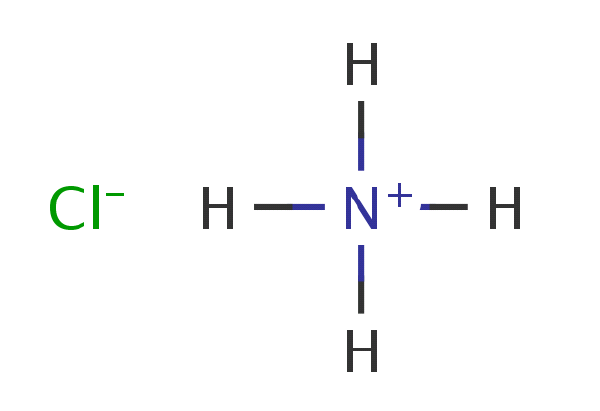Ammonium Chloride Formula: Properties, Uses and Examples
By A Mystery Man Writer
Last updated 17 Jul 2024

Ammonium chloride (NH4Cl) that is also known as the ‘sal ammoniac’ is the salt of the ammonia and the hydrogen chloride. Its most common uses are as a nitrogen supply in the fertilizers and as an electrolyte in the dry cells. Ammonium Chloride Formula will help you understand this better.

Algae Chemistry Pool & Spa News

CH150: Chapter 4 - Covalent Bonds and Molecular Compounds - Chemistry
Ethylammonium chloride, C2H8ClN
Ammonium chloride, H4ClN

Review of Ammonium Chloride–Water Solution Properties

Chemical Properties of Alkalis – O Level Secondary Chemistry Tuition
Ammonium Chloride, NH4Cl
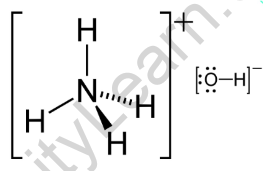
Ammonium Hydroxide Formula - Uses, Physical and Chemical Properties

Ammonium chloride NH₄Cl : Molecular Geometry - Hybridization
Recommended for you
You may also like